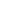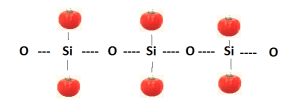Welcome to the first blog post on Polymers from FOC. If you begin following me on Twitter, you will read many insights on FOC’s different specialty products that I manage. I titled this first blog “everything you ever wanted to know in Randall’s World” but hope you enjoy many future views into my world of unique materials and solutions.
Now, Polymers….
Polymer (/ˈpɒlɨmər/ [2] [3])(Greek poly-, "many" + -mer, “parts”) is a large molecule composed of many repeated subunits. Schmatically rendered by chemists and engineers like this:
And artists such as Jackson Pollack like this:

Polymers range from familiar synthetic plastics such as polycarbonate to the natural biopolymer DNA. Silicone is the polymer that utilizes alternating silicon (Si) and oxygen (O) atoms as the repeating subunit to make flexible materials that caulk bath tubs and helped send Voyager to interstellar space.
Polymers owe their versatility to the large number of combinations and substitutions that can be made in the basic assembly of the molecule. In the case of silicones, the obvious feature of flexibility is attributed to the relatively large amount of rotation possible between the silicon and oxygen atoms that make up the molecule’s backbone (see diagram below). The length of this backbone causes the material to be high or low in viscosity: the longer the backbone the higher the viscosity due to greater entanglement of the long chain lengths (like spaghetti).
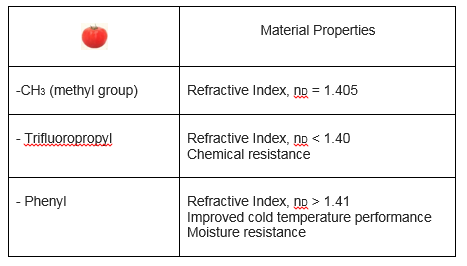
In the schematic the tomatoes represent placeholders for combinations of atoms that proffer further properties. The following table shows some of the different possibilities for the tomato groups and the resulting changes in the physical properties:
Every chemist I’ve ever known is an excellent cook, and anyone who’s cooked knows cooking is a lot like chemistry. So I like the food analogies. Here’s the easy take away message from the above. Polymers are like spaghetti - the spaghetti is the silicon-oxygen backbone, and the secret sauce is in the tomato groups.
This article is an original publication of Fiber Optic Center, Inc. It is shared publicly for educational and reference purposes to support learning and professional development within the fiber optics industry.
You are welcome to read, cite, or reference this material for non-commercial and educational purposes, as long as full credit is given to Fiber Optic Center, Inc. and the author.
Reuse, reproduction, or adaptation of this content — including rewriting, republishing, or incorporating it into new materials (such as websites, blogs, marketing text, technical guides, or AI-generated content) — is not permitted without prior written consent from Fiber Optic Center, Inc.
This material is protected by copyright law upon publication, even if not formally registered.
Use of this content for AI training, automated data extraction, or derivative content generation is prohibited.
Fiber Optic Center monitors and enforces the integrity of its intellectual property through digital identifiers and content tracking.
For more details, please refer to the Fiber Optic Center Content Use and Copyright policy.