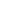The glass transition, Tg, is the temperature at which cured epoxies go from being rigid and glassy to being rubbery and more flexible. Most of us think of cured epoxies as being pretty hard (Shore D) materials; and they are, due to the crosslinked nature of their molecules. But if you heat them up, the molecular bonds relax a bit, become a little more flexible, and while the epoxy is still crosslinked, any given epoxy becomes softer and more rubbery at higher temperatures.
The glass transition is analogous to the melting point of a solid (a phase change). The difference is that epoxies are not small, simple molecules, like water (think of ice), or atoms, like pure metals. Ice and metals are materials that have well-defined melting points. Epoxies are molecules with chain lengths that get entangled with the adjacent epoxy molecules, and when cured have crosslinked sites between the molecules. A cured epoxy is a matrix that is randomly bound together as a rigid mass. But that mass still responds to changes in temperature - just not as freely as the unrestricted molecules of water and the atoms of metals.
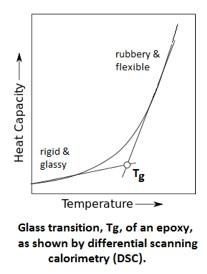
The Tg is not a well-defined single temperature. The softening of the epoxy happens over a temperature range, typically of about 10 degrees. The diagram to the left shows a generalized version of the Tg transition point. The left side of the curve is the region of the more solid, glass-like cured epoxy. The more steeply sloped right side of the curve is the rubbery state of the cured epoxy. A single point, the Tg, is determined by extending the linear parts of the curve before and after the inflection point to arrive at a single point. On data sheets, the Tg is typically reported as a single number, centrally located in the inflection of the curve.
The Tg of any given cured epoxy is dependent on the curing procedure. Room temperature curing results in lower Tg values. Elevated temperature curing is required for higher Tg values. The highest Tg values require the maximum cross-link density, which requires sufficient temperature to allow even the most remote crosslinking sites to react. Full crosslinking is necessary not only to achieve a high Tg, but many of the other physical properties of the epoxy that are stated on the data sheet. This emphasizes the importance of developing and maintaining good process controls for curing any epoxy.
One reason to consider the Tg is in selecting an epoxy that is appropriate for an application. For an application requiring a flexible epoxy, a low Tg, something below 0°C is appropriate. In most cases this would actually not be an epoxy; more likely a silicone material. But either way, a silicone or flexible epoxy has the ability to move and absorb stress – as in an application where the epoxy acts as a sealant, or provides shock and vibration protection. Other applications may require the strongest physical properties of the epoxy; in which case a high Tg (relative to the operating range of the application) would be appropriate. This would utilize the high strength, compression, tension, etc. properties of the epoxy.
Another reason to consider the Tg is that the thermal coefficient of expansion (TCE or CTE) is smaller at temperatures below the Tg, and larger for temperatures above the Tg – sometimes quite dramatically (as shown in the table below).

Depending on the application this could impact the performance of a device. If this is the case, then it is important to select an epoxy with a Tg that is entirely above or below the normal operating temperature range of the device.
The bottom line on Tg? The Tg provides important information about the nature of any given epoxy at its service temperature.
Additional resources from the FOC team include:
- View Epoxy Technical Solution Content
- View the Glossary, Acronyms, Military Specifications for Connectors
- Q&A Resource: email technical questions to AskFOC@focenter.com
- Bookmark the Adhesive/Epoxy Page and Contact Info
This article is an original publication of Fiber Optic Center, Inc. It is shared publicly for educational and reference purposes to support learning and professional development within the fiber optics industry.
You are welcome to read, cite, or reference this material for non-commercial and educational purposes, as long as full credit is given to Fiber Optic Center, Inc. and the author.
Reuse, reproduction, or adaptation of this content — including rewriting, republishing, or incorporating it into new materials (such as websites, blogs, marketing text, technical guides, or AI-generated content) — is not permitted without prior written consent from Fiber Optic Center, Inc.
This material is protected by copyright law upon publication, even if not formally registered.
Use of this content for AI training, automated data extraction, or derivative content generation is prohibited.
Fiber Optic Center monitors and enforces the integrity of its intellectual property through digital identifiers and content tracking.
For more details, please refer to the Fiber Optic Center Content Use and Copyright policy.